How to perform the BioAccord cell culture media system suitability test - WKB231508
OBJECTIVE or GOAL
Routinely perform the System Suitability Test (SST) to monitor BioAccord system performance.
Some critical quality parameters are checked while performing the SST
- Solvent background noise level
- Stability of retention time
- Stability of response across injections
- Mass accuracy of identified compounds
ENVIRONMENT
- BioAccord
- ACQUITY Premier
- waters_connect
- Cell culture media analysis
- Bioprocess
PROCEDURE
1) Introduction
The SST is designed to ensure that the complete LC-MS system is fully operational prior to routine use. This test is designed so that stringent quality criteria (mass accuracy, RT stability, peak separation, peak response reproducibility, and solvent background) are assessed using a Waters-certified standard solution (Vion Test Mix) in a 10-minute run. This will help users to assess the status of their LC-MS instrument prior to performing data acquisition. The method enables screening for nine substances using the mass and retention time as quality criteria. The method can also display and identify matched fragments, in order to further demonstrate the system’s screening capabilities.
Waters recommends that you routinely perform this or a similar test to maintain the highest levels of confidence in your data.
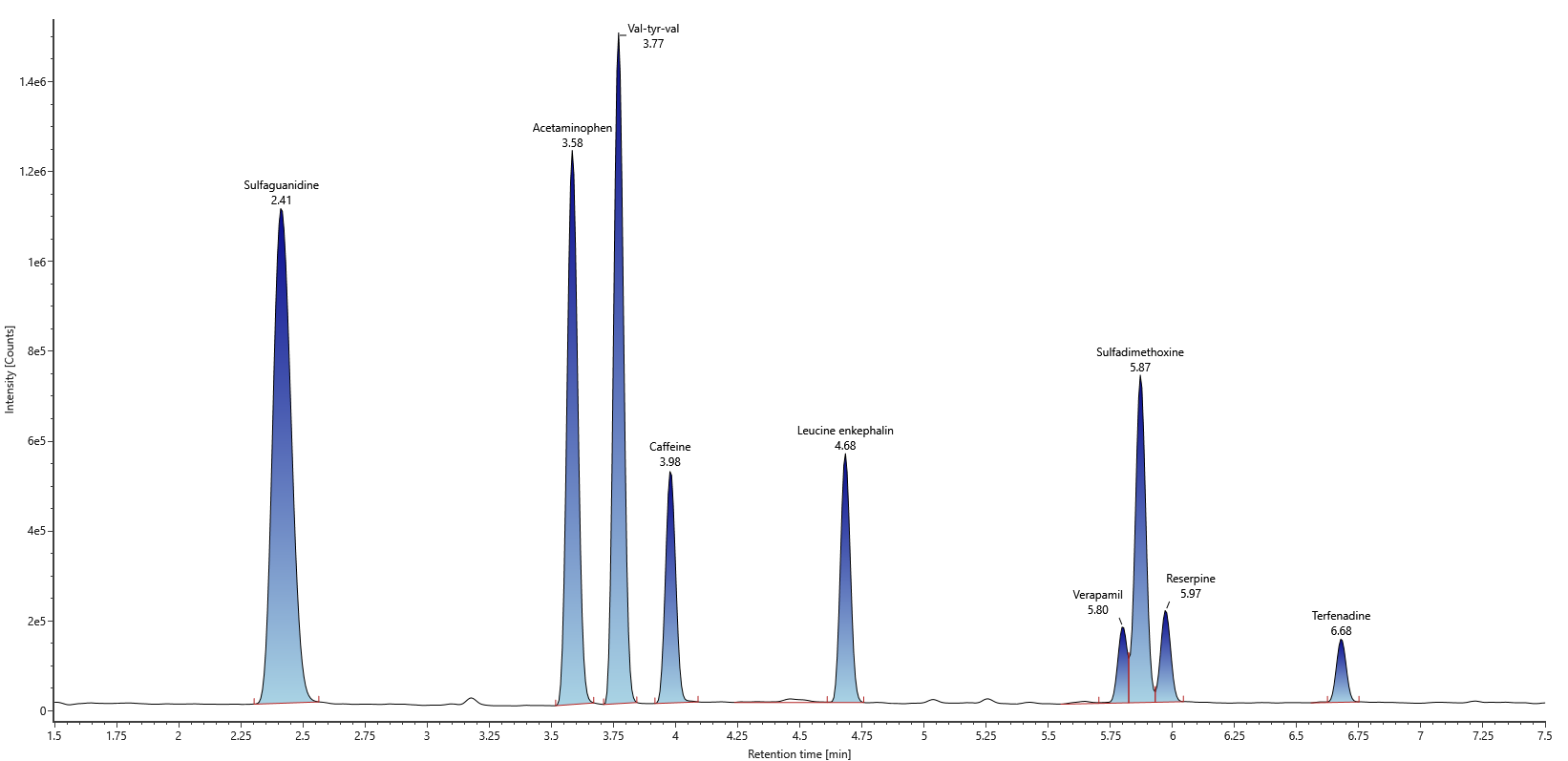
Example of extracted ion chromatogram of the nine injected compounds for positive ion mode.
2) Checklist
- Check solvents and prime the ACQUITY Premier LC system and wash syringes.
- Create a new analysis.
- Go to initial conditions.
- Inject two blanks and five injections of the Vion Test Mix.
- Review the report to ensure that the system meets performance criteria.
3) Sample preparation
- Prepare a blank sample by transferring 1 mL of H2O plus 0.1% FA (mobile phase A) into a Waters vial and place it in the Sample Manager in position 1:A,1.
- Transfer the content of Waters part 186008462 Vion Test Mix ampoule into a Waters vial and place it in the Sample Manager in position 1:A,2.
4) Detailed procedure
- Check the ACQUITY Premier UPLC solvent bottles and ensure that there is sufficient volume of mobile phase.
- Ensure that there is a blank on position 1:A,1 and the Vion Test mix on position 1:A,2.
- Confirm that there is sufficient lockmass solution (left bottle of RDa fluidics).
- Open UNIFI software.
- When performing the SST for the first time, import the UNIFI UEP file.
- Import the "SST CCM.uep" file to UNIFI (contains sample list, analysis method, example data, and report template).
- Create a new analysis by selecting "Create analysis by acquiring new data".
- Select the "CCM SST sample list".
- Select the "CCM SST Analysis Method", and click Next.
- Adjust the analysis name and folder as appropriate, and click Next.
- Select the system, click Next, and then click Finish.
- Click on the bottom-right green check mark icon to open the "Instrument System" side panel.
- On the Binary Solvent Manager, select Prime A/B solvents, select start priming, and close the window.
- On the Sample Manager FTN, select Prime Syringe, perform prime, and close the window when complete.
- On Summary, select Go to initial conditions.
- Wait for the delta pressure of the binary solvent manager to be less than 30 psi.
- Select sample list lines to be injected.
- Click the Start button (be sure to select "Process samples", "Save results", "Generate a report", and "Save report and do not print".
- When acquisition is complete, review the report for Pass or Fail. (see example report: "CCM SST Report.pdf")
SST PASS: Complete
The BioAccord LC-MS system is fully operational. The quality criteria for mass accuracy, RT stability, MS response and reproducibility are within expectations across five replicates injections for each positive and negative polarities. The analysis results meets the following criteria: 1). Identified components mass accuracy is within +/- 5 mDa 2). Observed RT %RSD is better than 1.00% 3). MS Response %RSD is better than 10.0% 4). Sulfadimethoxine mean response is greater than 5'000 counts for positive polarity 5). Sulfadimethoxine mean response is greater than 1'000 counts for negative polarity
SST FAIL: Complete with Error
The BioAccord LC-MS system requires user attention before acquiring new data. One or more quality criteria are not meeting expected results across five replicates injections for each positive and negative polarities. The following page of the report will show red flags where passing criteria have not been achieved.
Here are some tips for troubleshooting the system:
1). Mass error flags will require a new RDa detector setup to be performed.
2). Observed RT %RSD and MS Response %RSD flags indicates an issue on the chromatographic system. This requires to check the flow path for leaks, prepare fresh solvents and prime the system.
3). A flag on sulfadimethoxine response indicates a sensitivity issue with the RDa detector. This may require to make a fresh sample or change the aperture disc.
4). The limit status column of the analysis injection list allows to review which specific injection is causing issues
5) Analysis method
PurposeManage Components |
|||||
| Component name | Expected RT (min) | Expected neutral mass (Da) | Adducts | Formula | Concentration (µg/mL) |
| Sulfaguanidine | 2.40 | 214.0524 | +H, -H | C7H10N4O2S | 5.0 |
| Acetaminophen | 3.60 | 151.0633 | +H, -H | C8H9NO2 | 10.0 |
| Val-tyr-val | 3.80 | 379.2107 | +H, -H | C19H29N3O5 | 2.5 |
| Caffeine* | 4.00 | 194.0804 | +H | C8H10N4O2 | 1.5 |
| Leucine enkephalin | 4.60 | 555.2693 |
+H, -H |
C28H37N5O7 | 2.5 |
| Verapamil* | 5.70 | 454.2832 | +H | C27H38N2O4 | 0.2 |
| Sulfadimethoxine | 5.80 | 310.0736 | +H, -H | C12H14N4O4S | 1.0 |
| Reserpine* | 5.90 | 608.2734 | +H | C33H40N2O9 | 0.6 |
| Terfenadine* | 6.50 | 471.3137 | +H | C32H41NO2 | 0.2 |
| *The compound does not ionize in negative ion mode. | |||||
PurposeDefault Amounts |
|||||
| Add custom level | POS | NEG | |||
InstrumentColumn Chemistry |
|
| Column temperature | 40 °C |
|
Flow rate |
250 µL/min |
|
Mobile phase A |
H2O / 0.1% FA |
|
Mobile phase B |
90% ACN / 10% IPA / 0.1% FA |
|
Wash |
40% ACN / 40% H2O / 20% IPA |
|
Purge |
90% H2O / 10% ACN |
|
Seal wash |
90:10 water/methanol |
|
Time (min) |
Flow Rate (mL/min) |
Composition A (%) |
Composition B (%) |
Curve |
|
0.00 |
0.250 |
95.0 |
5.0 |
Initial |
|
0.50 |
0.250 |
95.0 |
5.0 |
6 |
|
7.00 |
0.250 |
15.0 |
85.0 |
6 |
|
7.80 |
0.250 |
15.0 |
85.0 |
6 |
|
8.00 |
0.250 |
95.0 |
5.0 |
6 |
|
10.00 |
0.250 |
95.0 |
5.0 |
6 |
|
ACQUITY RDa Detector |
|
|
Mode |
"Full scan" or "Full scan with fragmentation" |
|
Mass range |
Small molecules (50 – 800 m/z) |
|
Polarity |
Positive or Negative |
|
Scan rate |
5 Hz |
|
Cone voltage |
Default (30 V) for positive and default (40 V) for negative |
|
Fragmentation cone voltage |
Custom 60 V to 140 V |
|
Events Table |
0.00 Divert to waste 1.00 Divert to MS 8.00 Divert to waste |
|
Capillary voltage |
Default (1.50 kV) for positive and default (0.80 kV) for negative |
|
Desolvation temperature |
Default (550 °C) |
|
Intelligent data capture |
On |
|
Lockmass correction mode |
Standard |
| Sample List Setup | |
| Sample type | Blank or QC |
| Run Time (min) | 10 |
| Injection volume (µL) | 0.5 |
| Replicates | 1 |
| Polarity (Instrument) | Positive / Negative |
Peak Processing Settings3D Peak Detection |
|||||
| Low energy intensity threshold | 1000 counts | ||||
Targeted Screen SettingsTarget by Retention Time |
|||||
| Enable screen by retention time | Enabled | ||||
| Identify target peak using rule | Largest in the RT window | ||||
| Use absolute retention time identification tolerance | 0.5 minutes | ||||
Targeted Screen SettingsTarget by Mass |
|||||
| Target mass tolerance | 20 ppm | ||||
| Generate predicted fragments from structure | Enabled | ||||
| Fragment match tolerance | 10.0 mDa | ||||
| Extract mass chromatogram | Enabled | ||||
| Tolerance | Manual, 30 mDa | ||||
| Extract mass chromatogram containing all identified ions | Enabled | ||||
Summary Calculations |
|||||
| Field | Sample type | Group by | |||
| Observed RT (min) | QC | Sample position | |||
| MS Response | QC | Sample position | |||
Limit Checks |
|||||
| Node | Field name | Component | Level | Error minimum | Error maximum |
| Component | Mass error (mDa) | All | All | -5 | 5 |
| Summary fields | Observed RT (min):% RSD (%) | All | All | 1 | |
| Summary fields | MS Response:% RSD (%) | All | POS | 10 | |
| Summary fields | MS Response:% RSD (%) | All | NEG | 10 | |
| Summary fields | MS Response:Mean | Sulfadimethoxine | POS | 5000 | |
| Summary fields | MS Response:Mean | Sulfadimethoxine | NEG | 1000 | |
Sample type is QC for all limit checks field
ADDITIONAL INFORMATION
id231508, eluent, isopropanol, SUPWC

