How to test for HDX back exchange - WKB97409
OBJECTIVE or GOAL
Test for HDX back exchange.
Deuterons back exchange for protons, a common effect encountered on HDX, can have multiple causes: natural presence of water and hydrogen in quenching solution and buffers, mobile phases or buffers of unsuitable pH, higher than recommended temperatures used in the HDX manager or ESI source, or delays in injection and sample handling. As part of the System Suitability Test, back exchange can be evaluated by analyzing a tryptic digest of phosphorylase b (MassPREP Phosphorylase b Standard - 186002326).
ENVIRONMENT
- SYNAPT G2-S
- SYNAPT G2-Si
- SYNAPT XS
- Xevo G2-S
- Xevo G2-XS
PROCEDURE
The sample is reconstituted in 250 µl of 100% D2O, incubating overnight to allow all the peptides to be fully labeled.
- Then, a normal labeling experiment is carried out: equilibration, quenching, injection on the system (without pepsin column), and the percentage of the remaining labeling on the peptides is evaluated.
- Calculate the back-exchange by subtracting the measured deuterated value from the total number of amide hydrogens in that peptide. Slightly different back-exchange values could be observed compared with the back-exchange on the protein of interest. This is due to the tertiary structure of a protein and differences in amino acid sequence or position in the elution order. This should give you a good indication of back exchange of your system. The maximum deuteration is shown as scale y value in the Relative Uptake plot in DynamX. The expected value should be below 40%.
ADDITIONAL INFORMATION
Reference tryptic digest data: Online peptic digestion and trapping parameters were maintained at 70 µL/min for three minutes to reproduce expected back exchange. Deuteration exposure times were 30 minutes and 2 hours.
Peptide 235-242, approximately 70% of relative fractional uptake retained.
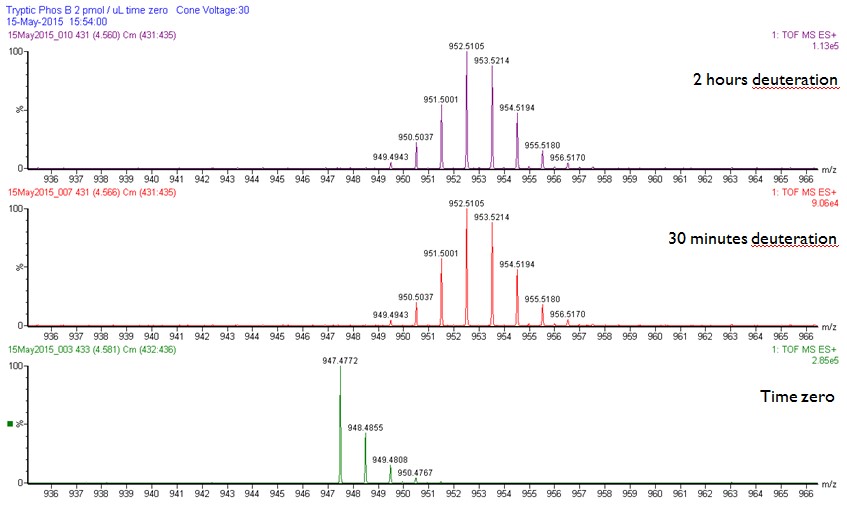
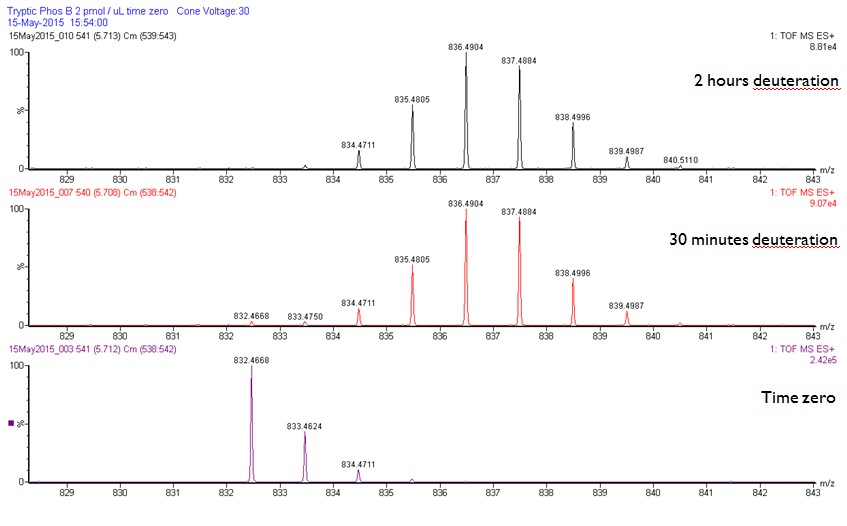
Peptide 248-255, approximately 60% of relative fractional uptake retained.
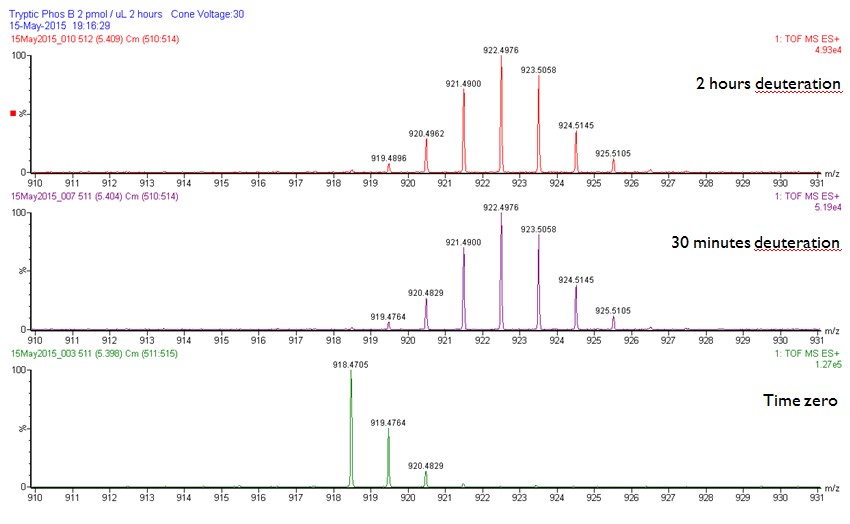
Peptide 364-370, approximately 64% of relative fractional uptake retained.
If the back exchange is found to be >40%, we recommend the following:
1. Prepare fresh solvents and buffers, adjusting very carefully the pH using a calibrated pH meter.
2. Check the temperature of the HDX Manager and verify that the cooling efficiency is high enough. Note that the back exchange will increase at high temperatures.
3. Verify the source temperature (80–100 ºC) and desolvation temperature (250 ºC).
4. Modify the MS settings, especially the ones related to the StepWave.
| Parameter | Optimized Values |
| Stepwave 1 wave height (V) | 30 |
| Stepwave 1 Velocity (m/s) | 300 |
| Stepwave 2 Wave height (V) | 30 |
| Stepwave 2 Velocity (m/s) | 200 |
| Stepwave 2 offset (V) | 15 |
| Diff Aperture 1 offset (V) | 3 |
| Diff Aperture 2 offset (V) | 0 |
| Stepwave rf Voltage (V) | 300 |
| Cone gas flow rate (L/h) | 90 |
Refer to Guttman et al., J. Am. Soc. Mass Spectrom. 2016, 27, 4, 662–668 for more details.

