How to avoid ammonium salt precipitation during the LC gradient - WKB14438
Article number: 14438
OBJECTIVE or GOAL
Avoid precipitation of ammonium salts from solvents during an LC gradient. (This can result in a decrease in mass spec sensitivity—due to potential blockage of the source cone aperture—and damage to columns and LC components.)
ENVIRONMENT
- Liquid chromatography modules such as ACQUITY QSM, BSM, and CM
- Mass spectrometers
- Ammonium salts such as ammonium formate, ammonium acetate, and ammonium phosphate
PROCEDURE
- Use adequate pH levels for the amount of dissolved ammonium salt, especially in the organic solvent. Typically, a more acidic pH can sustain a greater concentration of dissolved ammonium salt at high pressures. Addition of an acid such as formic acid can achieve this and aid ionization of analytes. See the table "Solubility of Various Buffers in Acetonitrile" below.
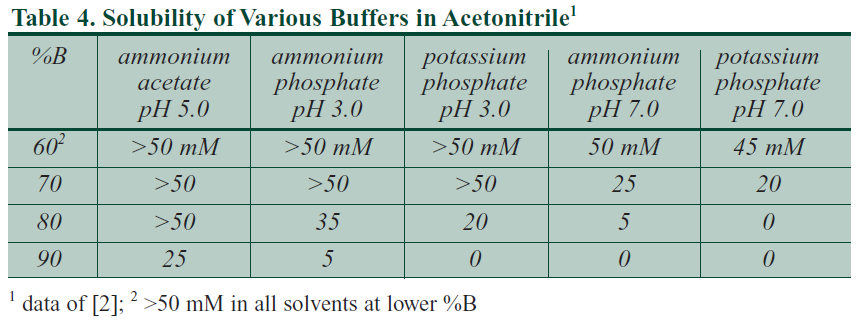
- Avoid having the ammonium salt present only in the aqueous solvent and absent from the organic solvent. This means that (1) the amount of ammonium salt varies during the LC gradient and (2) during the high pressure mixing of solvents in the LC mixer there is an increased risk of precipitation/"crashing out" of the ammonium salt.
- Aim to have a consistent level of ammonium salt in both the aqueous and organic solvents such that it remains constant throughout the LC gradient—for example, solvent (A) 10 mM ammonium salt in H2O + 0.01% formic acid; solvent (B) 10 mM ammonium salt in 90:10 acetonitrile/H2O + 0.01% formic acid. Note: for solvent (B), the ammonium salt must first be dissolved in the H2O portion, then the remaining volume of acetonitrile added to avoid precipitation.
ADDITIONAL INFORMATION

